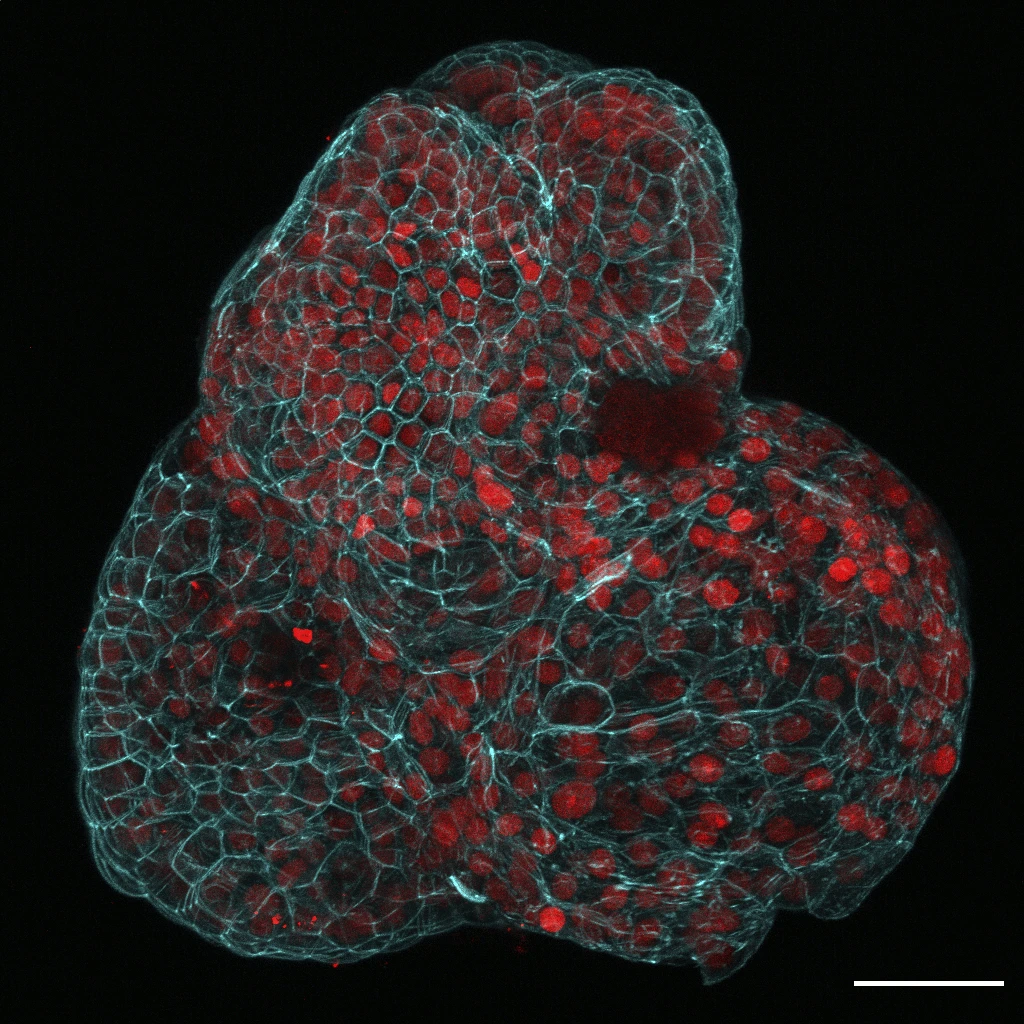
In a groundbreaking development within the realm of prenatal medicine, scientists have successfully engineered miniature organs, known as organoids, using cells extracted from the amniotic fluid enveloping a fetus in utero.
This innovative advancement is poised to revolutionize the field by offering unprecedented opportunities for testing novel medical interventions and comprehensively exploring the functionality of both healthy and diseased organs through these simplified structures.
A collaborative effort between the esteemed University College London and Great Ormond Street Hospital in the United Kingdom has yielded remarkable results.
By harvesting cells from amniotic fluid samples obtained during routine prenatal testing from 12 pregnancies, researchers have achieved a significant milestone.
Notably, this study marks the first instance of cultivating mini-organs from cells procured during ongoing pregnancies, showcasing the potential for a paradigm shift in prenatal healthcare.
Envisioning a future where this technique could enable healthcare providers to proactively monitor and address congenital conditions prenatally, as well as tailor personalized therapeutic interventions for unborn infants, the researchers are filled with optimism.
Mattia Gerli, a key contributor from University College London and an author of the study recently published in the prestigious journal Nature Medicine, expressed profound enthusiasm about the transformative prospects that lie ahead.
In their research, Gerli and his colleagues focused on the collection and analysis of tissue-specific stem cells that were shed by the fetus during pregnancy.
This shedding process is a natural occurrence that occurs as part of the fetal development process. Through their meticulous work, the scientists were able to identify the specific tissues from which these stem cells originated.
Their findings revealed the presence of stem cells from various organs including the lungs, kidneys, and intestines.
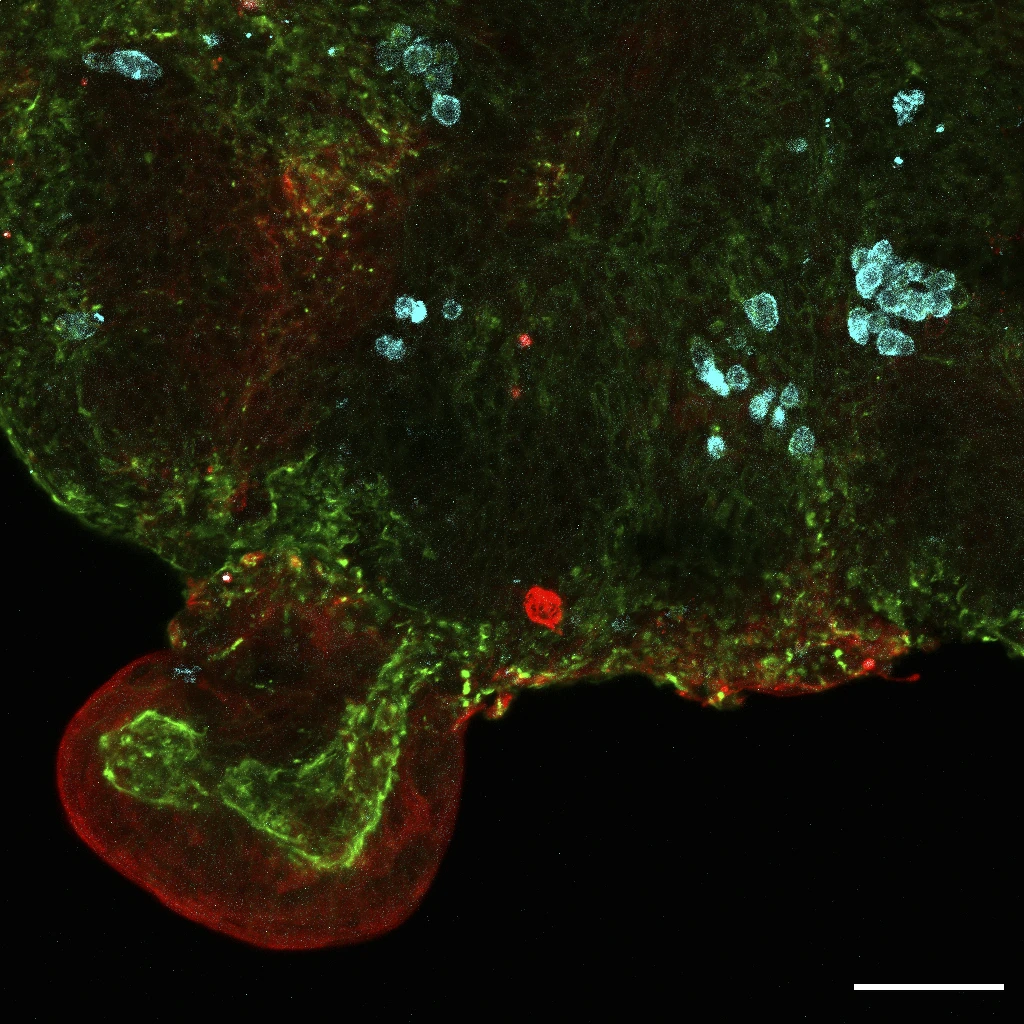
This discovery has significant implications for the field of regenerative medicine, as it provides valuable insights into the potential therapeutic applications of these tissue-specific stem cells.
By understanding the origins and properties of these cells, researchers can explore new avenues for developing innovative treatments for a wide range of medical conditions.
The identification of stem cells from vital organs such as the lungs, kidneys, and intestines opens up exciting possibilities for harnessing the regenerative potential of these cells to improve patient outcomes and advance medical science.
The utilization of mini-organs derived from adult stem cells has been a common practice in the past, as they closely mimic adult tissue or fetal tissue obtained post-abortion.
However, recent advancements in technology have allowed scientists to collect cells from amniotic fluid, circumventing regulations regarding the direct extraction of stem cells from fetal tissue.
This method enables researchers to obtain cells from fetuses in the later stages of pregnancy, providing valuable insights into human development and congenital diseases beyond the legal limits of termination in certain countries.
In the United Kingdom, termination of pregnancy is typically restricted to 22 weeks after conception, limiting access to fetal samples for research purposes.
Conversely, in the United States, regulations surrounding abortion and the use of fetal tissue for research vary by state.
Despite the legal complexities, the National Institutes of Health defines fetal tissue as originating from a deceased human embryo or fetus following a miscarriage, abortion, or stillbirth.
The ethical implications of using tissue from abortions have sparked debates within the scientific community and society at large.
Alta Charo, an emeritus professor of law and bioethics at the University of Wisconsin at Madison, highlights the controversial nature of this practice, underscoring the need for careful consideration of ethical standards in research involving fetal tissue.
In the realm of biomedical research and stem cell biology, the discourse surrounding the ethical implications of obtaining cells for study and therapeutic purposes has been a subject of intense scrutiny and debate.
Charo, a prominent figure not directly associated with the study in question, underscored the distinctiveness of the new methodology by asserting that acquiring cells from amniotic fluid, a substance routinely sampled for standard clinical procedures, does not introduce additional physical risks to either the developing fetus or the expectant mother.
This perspective sheds light on a pivotal aspect of the ethical considerations surrounding the utilization of such biological materials.
Furthermore, Dr. Arnold Kriegstein, a distinguished authority overseeing the Developmental and Stem Cell Biology Program at the esteemed University of California, San Francisco, emphasized the profound potential inherent in accessing cells through this method, highlighting the prospect of gaining valuable insights into the developmental trajectory of individual fetuses.
The notion of extracting cells from amniotic fluid, as articulated by Gerli, a researcher intimately familiar with the intricacies of this process, presents a unique window of opportunity for prenatal intervention and therapeutic strategies.
The timeline involved in cultivating miniature organs from cells sourced from amniotic fluid, typically spanning 4 to 6 weeks, not only allows for a comprehensive exploration of potential issues but also affords medical professionals a crucial window within which to address and rectify any identified anomalies through proactive prenatal interventions.
This convergence of perspectives from diverse experts underscores the multifaceted nature of the advancements in stem cell research and their far-reaching implications for prenatal care and therapeutic interventions.
The U.K. team, in collaboration with colleagues from Belgium, embarked on a study focusing on the practical application of their approach.
Their research delved into the development of infants afflicted with a congenital diaphragmatic hernia, a condition characterized by the displacement of organs like the liver and intestines into the chest due to a hole in the diaphragm.
This anomaly hampers the proper development of the lungs, leading to a mortality rate of approximately 30% among affected fetuses.
However, a ray of hope emerges as physicians can intervene by performing surgery on the fetus while still in the womb upon detecting the hernia.
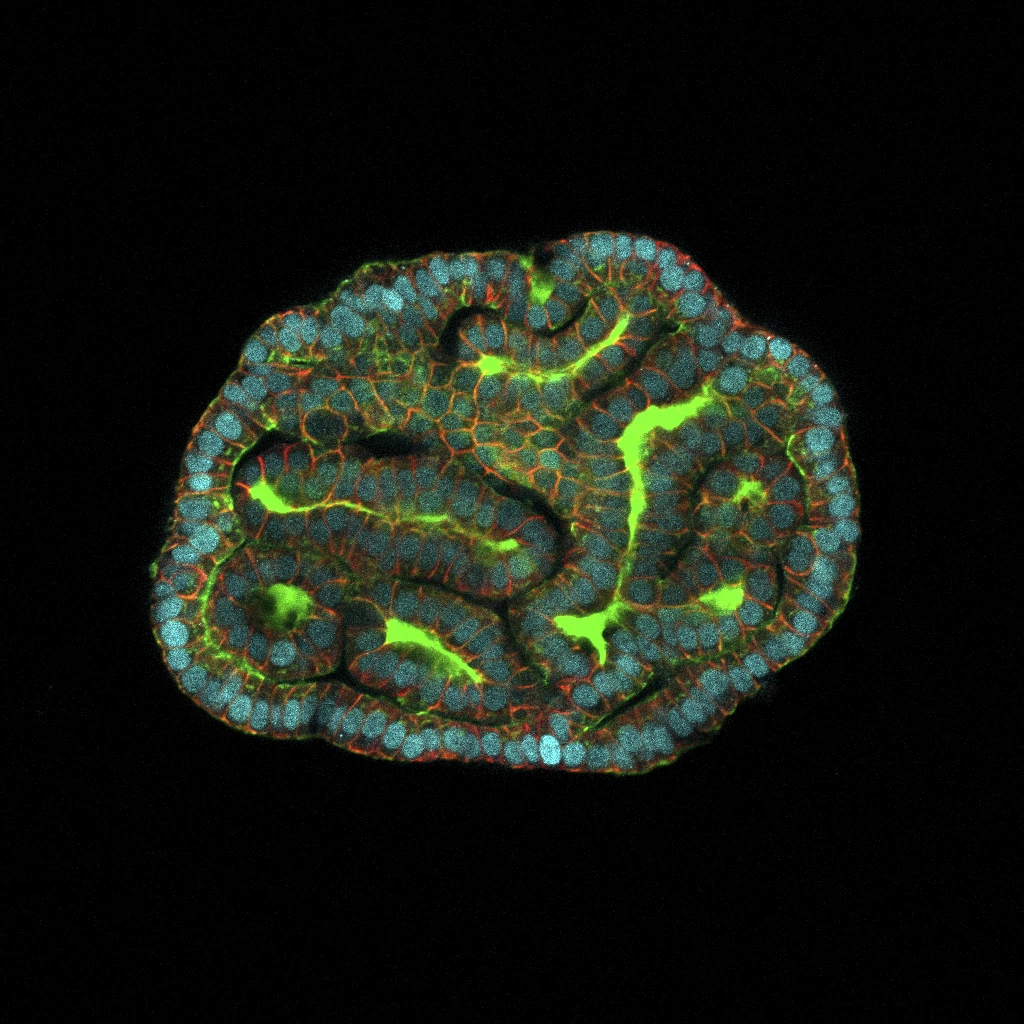
The researchers cultivated lung organoids using cells obtained from fetuses with the condition both pre and post-treatment, subsequently comparing them with organoids derived from healthy fetuses.
Dr. Paolo de Coppi, a study author affiliated with University College London and Great Ormond Street Hospital, highlighted the groundbreaking aspect of their findings.
By utilizing this method, they were able to evaluate the health status of the affected child even before birth. Dr. de Coppi emphasized the current limitation faced by medical professionals in providing families with accurate prognostic information following a prenatal diagnosis, attributing this challenge to the unique nature of each case.
The capability to analyze functioning prenatal miniorgans represents a crucial initial stride towards offering more precise prognoses and devising more efficacious treatment strategies for such conditions.